Indication Selection - Case Study
 A global pharma company asked Euretos to help in finding novel indications, with a focus on rare diseases, for a number of drugs already on the market. They had already created an initial list of indications which they wanted to assess but were also looking for novel indications with limited mention in literature but strong underlying biological data to support the target association. There was also severe time pressure and they wanted to conclude the initial selection process in 4 weeks.
A global pharma company asked Euretos to help in finding novel indications, with a focus on rare diseases, for a number of drugs already on the market. They had already created an initial list of indications which they wanted to assess but were also looking for novel indications with limited mention in literature but strong underlying biological data to support the target association. There was also severe time pressure and they wanted to conclude the initial selection process in 4 weeks.
Key Challenges
Especially with the very short timescales, there were significant challenges including:
Based on its many collaborations with leading pharma and biotech companies on indication selection, the company asked Euretos to support them in this endeavour.
Euretos Solution
For the project, Euretos was able to draw on it’s unique, AI-powered computational disease models that predict truly novel indication candidates for a target by:
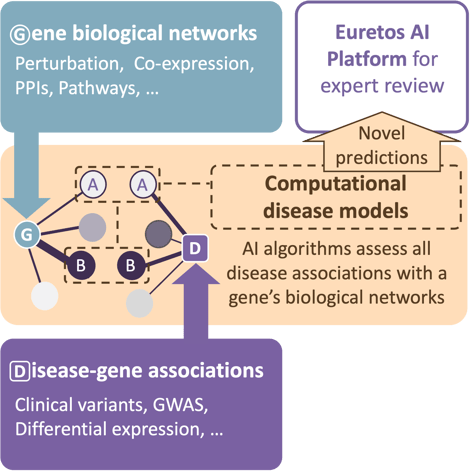
We knew from many previous projects that the ability to assess these predicted indications by target or disease experts is crucial to move a candidate forward in the selection process. We therefore provided the companies domain experts access to the Euretos AI Platform to independently evaluate the predicted indications through:
Results
The unique combination of novel predictions from the Euretos computational disease models with the ability for domain experts to assess these independently in the Euretos AI platform proved very effective. The company was able to build within a very short time frame the confidence necessary to decide which indications to move forward for further development.







Keep up with our latest news and events. Sign up for our newsletter.