The new Euretos Perspective article is here! This time triggered by a recent announcement by Sanofi that they are going ‘all in’ on artificial intelligence. In it, we discuss how we enable biopharmaceutical companies to go ‘all in’ on taking a data-driven, actionable approach to target discovery and assessment. Don't forget to Subscribe and stay tuned for more updates!
About two weeks ago, Sanofi came out with a major press release with the bold title: “Sanofi ‘all in’ on artificial intelligence and data science to speed up breakthroughs for patients”. In it, Sanofi announced its ambition to become the first pharma company powered by artificial intelligence at scale, giving their people the tools and technologies that focus on insights and allow them to make better everyday decisions.
This recent announcement fits a growing trend within biopharma where companies are investing heavily in AI-driven drug development. One of the key areas of investment is preclinical target selection where expectations are that using AI improves potential target identification by 20 to 30%. In order to remain competitive, it is imperative for biopharma companies to follow these examples and scale up their use of AI.
Euretos is a 10-year-old veteran in data-driven target discovery and we have one of the strongest track records in the industry. Our models contributed to approved drugs and active clinical trials in oncology, ulcerative colitis, NASH and epilepsy with 5 different biopharma clients. We combine biological knowledge graphs, predictive computational disease models and tailored target assessment workflows to enable biopharmaceutical companies to take a data-driven, actionable approach to target discovery and assessment.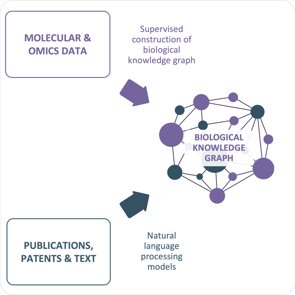
Computational disease models can provide insights in the disease mechanisms, underlying biological pathways, target molecules, and disease progression. In oncology, the target does not need to be directly linked to the disease pathology, but it should have a high potential for therapeutic intervention. Target discovery however is only the first step in such pre-clinical research program.
Once the target is identified, the project assesses the feasibility and appropriateness of different therapeutic modalities. This involves considering the characteristics of the target molecule, such as its location, size, and function, as well as the available technologies and expertise. For example, if the target is an intracellular protein, small molecules may be considered. If the target is a cell surface receptor, antibodies or cell-based therapies could be potential options.
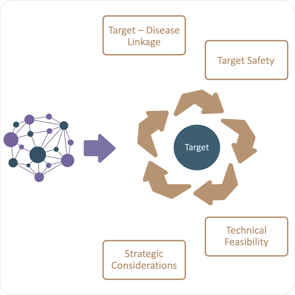
Such considerations are evaluated during Target Assessment. This step is typically very time consuming and is not always based on a clearly defined set of criteria. However, several successful biopharma companies have taken steps to improve the repeatability and productivity of target assessment. Euretos provides its Biological Knowledge Graph as the engine behind a data-driven target assessment process.
This enables companies to review existing preclinical and clinical data on similar therapeutic approaches used in similar diseases. This helps in evaluating the drugability, safety, efficacy, and potential limitations of each therapeutic modality. It can include the data on compound potency, selectivity, pharmacokinetics, and toxicity to determine the most promising approach.
In addition, the Euretos Knowledge Graph provides the intellectual property landscape related to different therapeutic approaches. It supports the assessments whether there are existing patents or competitive advantages in a particular modality that may affect the commercial viability of the drug candidate.
By considering these factors, along with scientific, clinical, and commercial insights, biopharmaceutical companies can make informed decisions about the most appropriate therapeutic approach for a given disease. It's important to note that the selection process may involve iterative cycles of evaluation, optimization, and refinement as new data and insights emerge during the drug development process.
By incorporating new data on drug response, safety profiles, adverse effects, or drug interactions into the computational disease models, this can help evaluate the overall benefit-risk profile of a potential therapeutic intervention. In addition, this enables the development of personalized medicine by identifying markers and individualized treatment approaches, and predicting treatment responses.
Author:
Aram Krol
CEO, Euretos
Subscribe to our mailing list and receive our quarterly updates!
Read our Terms and Conditions

Keep up with our latest news and events. Sign up for our newsletter.